Matter, the substance that makes up everything we interact with in the universe, exists in various forms known as the physical states of matter. Solid, liquid, gas, and plasma represent distinct phases characterized by differences in particle arrangement, energy levels, and behavior. Understanding these states is fundamental to grasping the properties of materials and their behavior under different conditions. This comprehensive article will delve into each state in detail, exploring their definitions, properties, and real-world applications.

1. Solid State:
Solids are perhaps the most familiar state of matter in our daily lives. They are characterized by their definite shape and volume, with particles that are closely packed together in a regular geometric arrangement. Here’s a closer look at the properties of solids:
-
Particle Arrangement:
- In solids, particles (atoms, ions, or molecules) are tightly packed and held together by strong intermolecular forces. This arrangement gives solids their rigidity and stability.
-
Shape and Volume:
- Solids have a fixed shape and volume. They maintain their shape because the particles are unable to move past one another to take on a new form.
-
Density and Compressibility:
- Solids typically have high density due to their closely packed particles. They are difficult to compress because the intermolecular forces between particles resist any attempt to reduce their volume.
-
Mechanical Properties:
- Solids can exhibit a wide range of mechanical properties, including hardness, brittleness, elasticity, and plasticity. These properties depend on factors such as the type of bonding between particles and the arrangement of atoms or molecules.
-
Examples:
- Common examples of solids include metals (such as iron and copper), minerals (like quartz and diamond), and organic solids (such as wood and plastics).
The study of solids is crucial in materials science and engineering, where researchers explore ways to manipulate their properties for applications ranging from construction materials to electronics and medical devices.
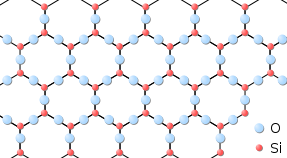
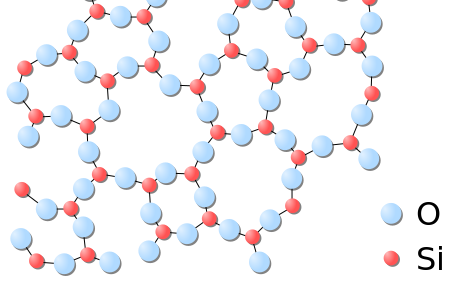
Applications:
Used in construction materials, electronics, medical devices, and various industrial applications where stability and strength are required.
Table of solid state:
| Property | Solid State |
|---|---|
| Particle Arrangement | Regular, tightly packed |
| Shape | Definite |
| Volume | Definite |
| Density | High |
| Compressibility | Low |
| Examples | Metals, minerals, plastics |
| Applications | Construction, electronics, industrial manufacturing |
2. Liquid State:
Liquids are another common state of matter, characterized by their ability to flow and take the shape of their container. Unlike solids, liquids do not have a fixed shape but do have a definite volume. Let’s examine their properties in detail:
-
Particle Arrangement:
- In liquids, particles are more loosely packed compared to solids, allowing them to move past each other. However, they still have significant intermolecular forces that keep them together.
-
Shape and Volume:
- Liquids take the shape of their container due to the ability of particles to flow and move freely. They have a definite volume because their particles are closely packed.
-
Density and Compressibility:
- Liquids have higher density compared to gases but lower than solids. They are relatively incompressible compared to gases due to the moderate intermolecular forces and close particle arrangement.
-
Surface Tension and Viscosity:
- Liquids exhibit surface tension (the cohesive force at the surface) and viscosity (resistance to flow), which vary depending on the type of liquid and temperature conditions.
-
Examples:
- Water, ethanol, and oil are common examples of liquids encountered in daily life. They exhibit typical liquid properties and behaviors.
Understanding liquids is crucial in fields such as chemistry, biology, and fluid mechanics, where their behavior under different conditions influences processes from chemical reactions to biological functions.
Applications:
Essential in biological systems, chemical reactions, and industrial processes where fluidity and conformability are required.
Table of liquid state:
| Property | Liquid State |
|---|---|
| Particle Arrangement | Close, mobile |
| Shape | Indefinite, takes the shape of the container |
| Volume | Definite |
| Density | Moderate |
| Compressibility | Low |
| Examples | Water, ethanol, oil |
| Applications | Hydration, chemical processes, lubrication |
3. Gaseous State:
Gases are characterized by their ability to expand to fill any container and lack a definite shape or volume. They exhibit unique properties distinct from solids and liquids:
-
Particle Arrangement:
- Gas particles are spaced far apart and move freely at high speeds. They have weak intermolecular forces, leading to random motion and a lack of fixed arrangement.
-
Shape and Volume:
- Gases expand to fill their container, taking its shape. They have no fixed volume because the particles are widely spaced and move independently.
-
Density and Compressibility:
- Gases have low density compared to solids and liquids because of their widely spaced particles. They are highly compressible due to the large gaps between particles.
-
Pressure and Temperature:
- The behavior of gases is governed by principles like Boyle’s Law (pressure-volume relationship) and Charles’s Law (temperature-volume relationship), which describe how gases respond to changes in pressure and temperature.
-
Examples:
- Air (a mixture of gases), helium, and carbon dioxide are examples of gases commonly encountered in everyday life.
The study of gases is essential in fields such as atmospheric science, thermodynamics, and industrial processes, where their behavior under different conditions influences everything from weather patterns to the efficiency of industrial reactions.

Applications:
Used in weather forecasting, industrial processes (e.g., manufacturing, refrigeration), and propulsion systems.
Table of gaseous state:
Property |
Gaseous State |
|---|---|
| Particle Arrangement | Widely spaced, fast-moving |
| Shape | Indefinite, fill the container |
| Volume | Indefinite |
| Density | Low |
| Compressibility | High |
| Examples | Air, helium, carbon dioxide |
| Applications | Weather forecasting, industrial processes, propulsion |
4. Plasma State:
Plasma is often considered the fourth state of matter, distinct from solids, liquids, and gases. It is characterized by ionized particles and exhibits unique properties:
-
Ionization:
- Plasma consists of ionized gas particles—atoms or molecules where electrons have been stripped away, resulting in positively charged ions and free electrons.
-
Behavior:
- Plasma is electrically conductive and responds strongly to electromagnetic fields. It does not have a definite shape or volume and can be influenced by electric and magnetic fields.
-
Occurrence:
- While plasma is rare on Earth, it is abundant in the universe, and found in stars (like the Sun), lightning, and some types of artificial lighting (such as neon signs and fluorescent lights).
-
Examples:
- Solar wind, lightning, and auroras are natural examples of plasma, while plasma displays, fusion reactors, and certain industrial processes utilize plasma technology.
Plasma research is critical in fields such as astrophysics, fusion energy research, and materials processing, where its unique properties offer opportunities for technological advancement and scientific discovery.

Applications:
Used in plasma displays, fusion research, and industrial processes such as materials processing and sterilization.
Table of plasma state:
| Property | Plasma State |
|---|---|
| Particle Arrangement | Ionized particles |
| Shape | Indefinite |
| Volume | Indefinite |
| Conductivity | Electrically conductive |
| Examples | Solar wind, lightning, auroras |
| Applications | Plasma displays, fusion research, materials processing |
Differences Among States:
| Property | Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Particle Arrangement | Regular, fixed | Close, mobile | Widely spaced, fast-moving | Ionized particles |
| Shape | Definite | Indefinite | Indefinite | Indefinite |
| Volume | Definite | Definite | Indefinite | Indefinite |
| Density | High | Moderate | Low | Low |
| Compressibility | Low | Low | High | High |
| Conductivity | Insulator | Insulator | Insulator | Conductor |
Conclusion:
The physical states of matter—solid, liquid, gas, and plasma—form the foundation of our understanding of the universe and its countless phenomena. From the rigidity of solids to the flow of liquids, the expansiveness of gases, and the electrically charged nature of plasma, each state offers unique insights into material behavior under different conditions.
By studying these states, scientists and engineers can develop new materials, improve technologies, and deepen our understanding of natural and artificial processes—from the microscopic world of atoms to the vastness of space. As research continues to expand our knowledge, the physical states of matter remain a cornerstone of scientific exploration, innovation, and technological progress.
